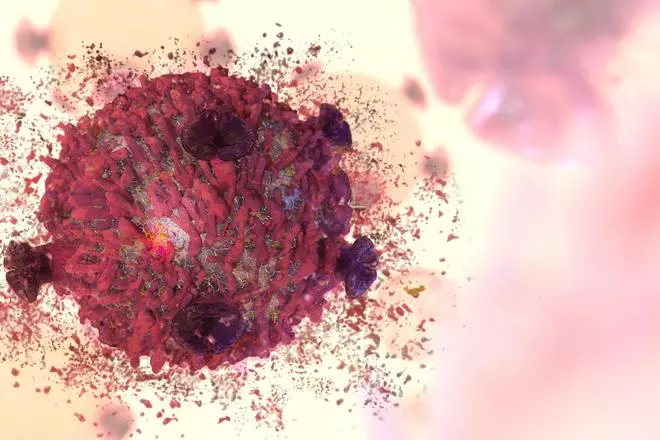
Conventional therapies in cancer treatment face challenges in delivery of drugs in the body and just the quantity needed, due to the toxic nature of the medicines used that have unwanted side effects.
Nanomaterials have ‘enormous’ potential in cancer treatment, says Dr V Ganesh Kumar, Scientist & Associate Professor at the Centre for Ocean Research (COR), Sathyabama Institute of Science and Technology. “They help alter the drug toxicity profile with enhanced surface characteristics which can diffuse inside the tumour cells. They deliver an optimal concentration of nano drugs at tumour sites and reduce toxicity,” he says.
Dr. Kumar is the corresponding author for a review paper to be published by Elsevier in the January 2023 volume of its Nanomedicine: Nanotechnology, Biology and Medicine journal. The other authors of the review include Ph.D scholar CG Anjali Das, Junior Research Fellow, pursuing research in anti-cancer studies.
In nanomedicine, three kinds of nanomaterials are studied predominantly – organic, inorganic and hybrid involving both. They include dendrimers, liposomes and exosomes, quantum dots, fullerenes, polymeric micelles, nanoemulsions, RNA nanoparticles and nanotubes.
Examples of organic molecules are dendrimers — which have a branched structure — and liposomes that are akin to lipids, with each having a property that helps inhibit a cancer cell.
Drug carriers
Nanoparticles (particles less than 100nm in length) trump traditional drugs and their delivery mechanisms across three areas: surface characteristics, ability to alter the toxicity of active cancer cells, and tumour specific constituents.
An inorganic nanoparticle such as gold, silver or platinum, acts as a drug carrier. How exactly does the drug delivery take place? “The electrostatic forces between adjacent molecules help in the drug delivery to the tumour site,” he says.
Nanomaterials are useful especially when only a specific amount of drug needs to be delivered and anything in excess would only cause side-effects of the drug. When nanodrugs reach the cancer site, they inactivate the multiplying property of the cancer cell by mutating the ‘signalling pathways’ that aid the proliferation of cells, explains Dr Kumar.
How does the field of medicine narrow down upon tumour-specific constituents of nanomaterials? Dr. Kumar cites the example of the organic nanomaterial, liposome. “The quantum of nano-drugs is at a ‘trace’ level. “We always study bio-compatibility. That is, how much of a drug can a cancer patient bear, what reactions the patient shows to such drugs...”
Liposomes disseminates inside a cell and easily disintegrates with time. In the use of metals, there is always the danger of accumulation of the drug residue which could have an impact on the patient in future.
Liposomes are drug delivery molecules that play a vital role in pharmaceuticals and in the biomedical arena. Marine-derived liposomes act as drugs. They are organic nanomaterials that are effective in drug delivery due to their biocompatibility, enhanced drug solubility, and their non-toxic nature, in addition to being biodegradable. Liposomes can be derived from plants and marine sources.
Does that mean that liposomes are the preferred category of nanomaterials? According to Dr Kumar, researchers are working on both organic and inorganic nanomaterials. “Both are advantageous in their respective application modes. While liposomes are applicable in a higher number of treatment cases than other nanomaterials, it is not an individual decision. For every patient, an oncology board will decide depending on the case history down to the level of blood pH level of the patient. Some kinds of cancers in certain patients may be more susceptible to dendrimers than liposomes. In other cases, oncologists may suggest the treatment with radiation, no material — nano or other kinds — may be required.”
Tumour-specific
If inorganic nanomaterials are invariably transporters of drugs and their organic cousins are drugs themselves, then why is the former in consideration at all? Isn’t it easier to just deliver the drug rather than mount it at nano-levels on a transporter and then aim for the cancer site? Kumar says, “The doctors may opt for controlled release. That is, the drug must neither stay at the site for a long time nor disintegrate quickly. And it must get active only at the cancer site; in such instances, you need a carrier for targeted drug delivery.”
Listing a host of nanoparticles that have been effective in treatment of cancer, the review showed, for example, that spherical gold nanoparticles synthesised in the lab using marine bacteria Vibrio alginolyticus were effective in decreasing cell viability in breast cancer cell line.
The review paper also lists 12 nanomedicines that have been clinically approved for the treatment of cancer. Not surprisingly, liposomes form the bulk of the mentions — in 4 cases — and are used in the treatment of pancreatic cancer, acute lymphoblastic leukemia, acute myeloid leukemia and osteosarcoma.







Comments
Comments have to be in English, and in full sentences. They cannot be abusive or personal. Please abide by our community guidelines for posting your comments.
We have migrated to a new commenting platform. If you are already a registered user of TheHindu Businessline and logged in, you may continue to engage with our articles. If you do not have an account please register and login to post comments. Users can access their older comments by logging into their accounts on Vuukle.